F.D.A. Tells J.&J. to 60 Million Doses Made at Can’t Be Used
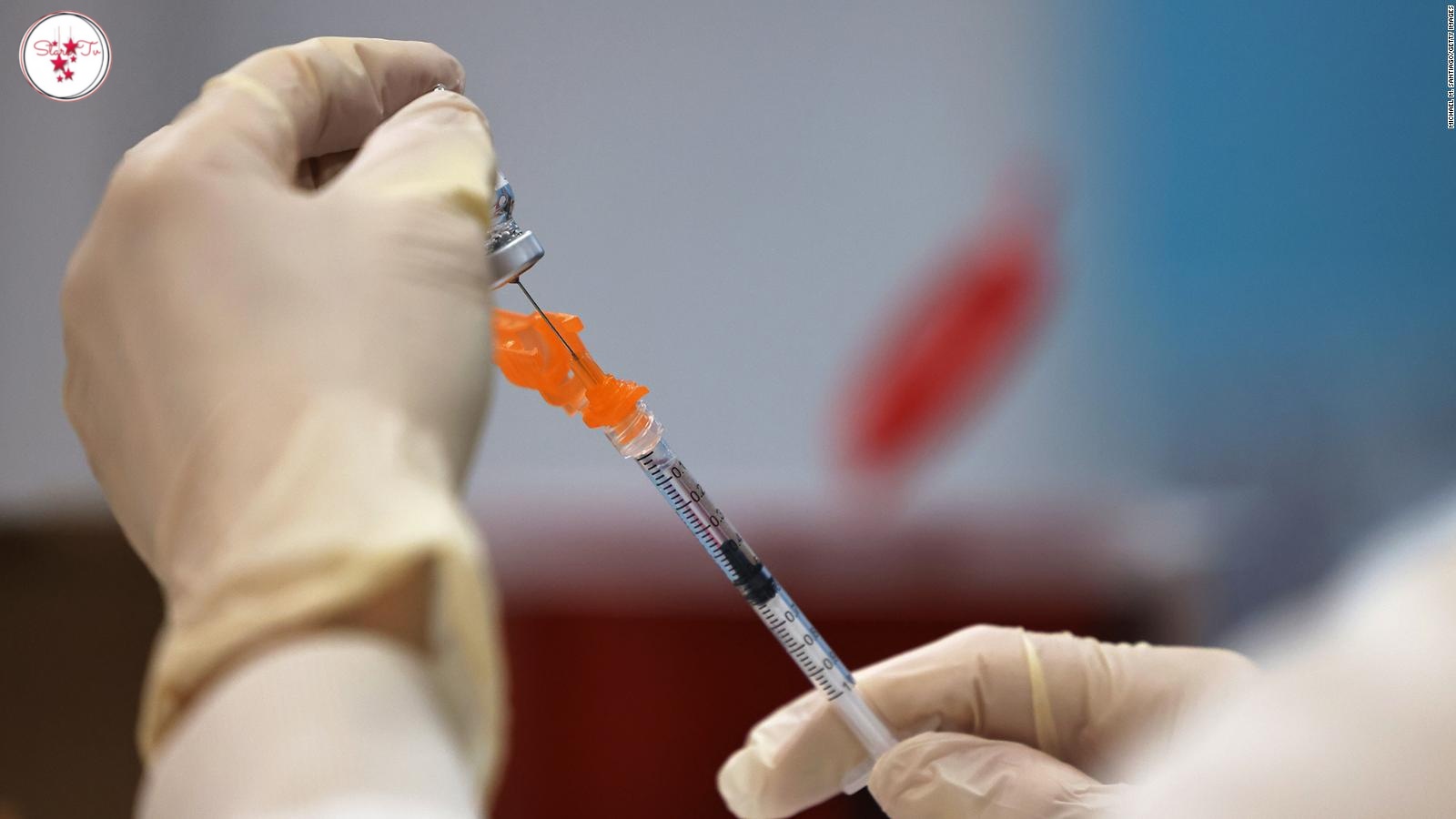
Federal regulators have advised Johnson & Johnson that about 60 million doses of its coronavirus vaccine produced at a afflicted Baltimore manufacturing unit can't be used due to the fact of viable contamination, in accordance to people acquainted with the situation.
The Food and Drug Administration plans to enable about 10 million doses to be allotted in the United States or dispatched to different countries, however with a warning that regulators can't warranty that Emergent BioSolutions, the business enterprise that operates the plant, observed appropriate manufacturing practices.
The employer has not yet determined whether or not Emergent can reopen the factory, which has been closed for two months due to the fact of regulatory concerns, the people said.
The Johnson & Johnson doses administered in the United States so far had been manufactured at the firm’s plant in the Netherlands, not through Emergent. For weeks the F.D.A. has been attempting to parent out what to do about at least one hundred seventy million doses of vaccine that had been left in limbo after the discovery of a primary production mishap involving two vaccines manufactured at the Baltimore factory.
More than a hundred million doses of Johnson & Johnson and at least 70 million doses of AstraZeneca had been put on hold after Emergent found in March that its employees had contaminated a batch of Johnson & Johnson’s vaccine with a key ingredient used to produce AstraZeneca’s. Federal officials then ordered the plant to pause production, stripped Emergent of its duty to produce AstraZeneca’s vaccine and steered Johnson & Johnson to assert direct control over the manufacturing of its vaccine there.
Johnson & Johnson’s vaccine was once viewed a achievable game-changer in the nation’s vaccine inventory due to the fact it required solely one shot and was specifically beneficial in prone communities. But the federal government now has an enough supply of the vaccines from Pfizer-BioNTech and Moderna, the two other federally licensed vaccine developers, and no longer desires Johnson & Johnson’s supply.
Still, the loss of 60 million Johnson & Johnson doses places a dent in the Biden administration’s plan to distribute vaccines to different nations that are still in the grip of the pandemic. The administration had been counting on sharing doses of each Johnson & Johnson and AstraZeneca however had to prolong its plan whilst the F.D.A. finished a review of the facility.
After he arrived in Britain for the Group of 7 summit this week, President Biden announced he had discovered some other source for donations. Pfizer-BioNTech has now agreed to sell his administration five hundred million doses at value for donation to low and lower-middle income nations over the next year. The World Health Organization estimates that eleven billion doses are wanted globally to stamp out the epidemic.
The F.D.A.’s action is disappointing information for Emergent and Johnson & Johnson, which employed the company as a subcontractor. Inspectors are nonetheless reviewing the plant and are not predicted to figure out whether or not the organization can reopen it till later this month, in accordance to human beings acquainted with the situation. Regulators are additionally persevering with to forged doubt on whether or not the company, which has been paid lots of hundreds of thousands of greenbacks via the federal authorities to manufacture coronavirus vaccines, adhered to manufacturing standards.
The agency’s plan to permit 10 million doses to be used in the United States or overseas with a warning is really uncommon for a product below emergency authorization, specialists said. Regulators have the discretion to take that action if the drugs are badly wished and in quick supply, they said.





.jpeg)



.jpeg)